Primary HPV
Screening Initiative
About The Initiative
In 2020, the American Cancer Society released the updated Cervical Cancer Screening Guideline supporting the transition to primary HPV screening as the preferred method for cervical cancer screening. Primary HPV screening every 5 years has been shown to be a more effective approach to cervical cancer screening, but the transition from less effective options has been slow. In the fall of 2021, the American Cancer Society’s Primary HPV Screening Initiative set out to support the transition to primary HPV screening as the dominant screening approach in the United States. With nearly 100 expert volunteers on six workgroups and a Steering Committee, this initiative goes beyond guideline development and into guideline implementation.
The Initiative fills a key implementation gap in cervical cancer screening and management in the U.S. and is a crucial step in successful primary HPV screening adoption and guideline alignment across organizations. Achieving these objectives will provide a critical infrastructure to support continuing advancements in cervical cancer prevention, early detection, and elimination in the coming years.
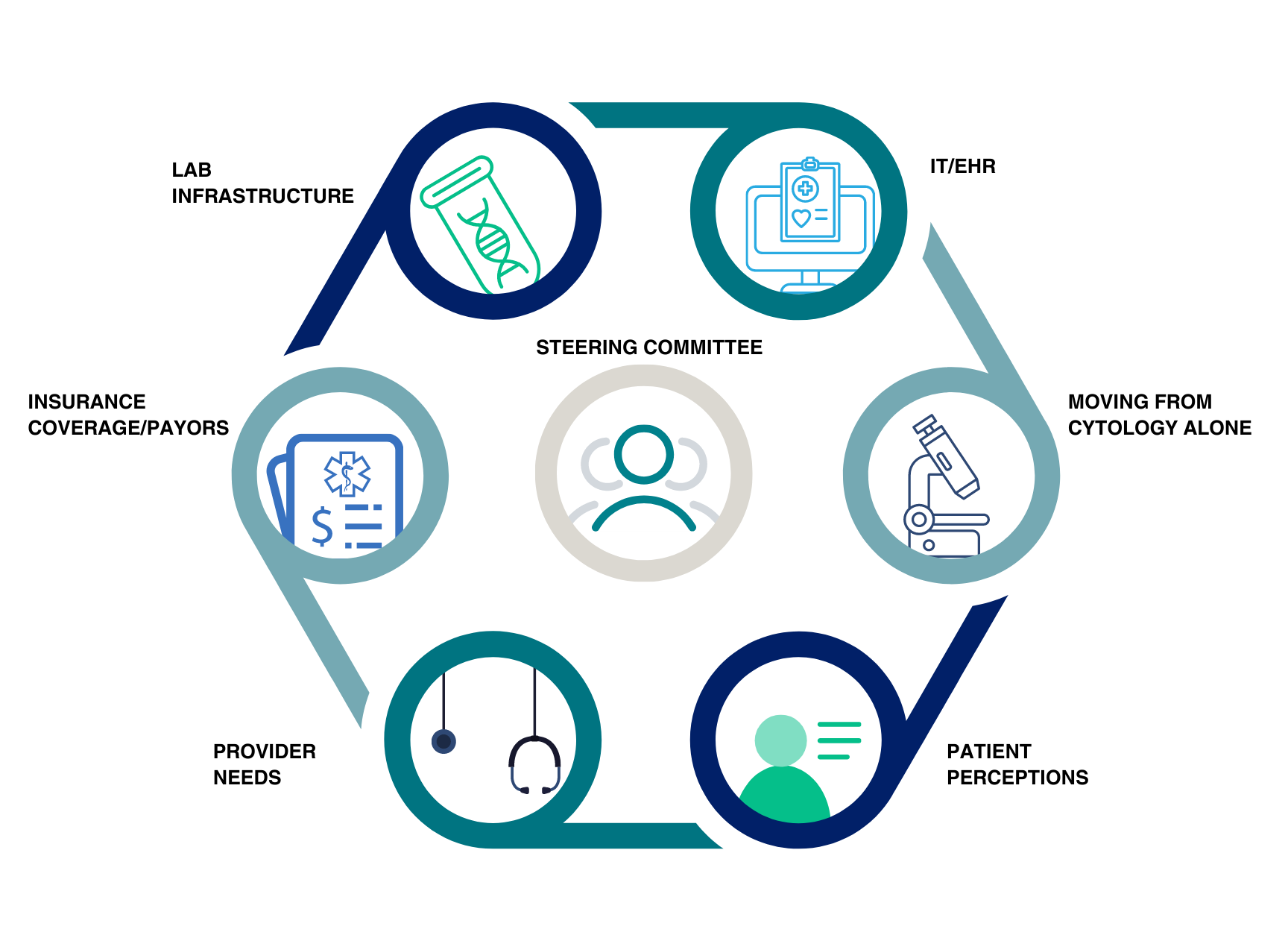
Initiative Workgroup Structure
Resources
Resources are available for primary HPV screening implementation support.
The Primary HPV Screening Iniative Intersects with:
Synergy with the ACS NRTCC
The American Cancer Society National Roundtable on Cervical Cancer (ACS NRTCC) is focused on the elimination of cervical cancer by improving prevention, screening, treatment, and survivorship. The Primary HPV Screening Initiative addresses implementation challenges that face the future cervical cancer screening paradigm, thus positively contributing to the ACS NRTCC’s aim to improve screening. This initiative plays a key role in connecting screening guideline adoption to practice changes, resource development, and advances that the Roundtable will promote. This initiative has also established and fostered crucial relationships that will be carried forward on the Roundtable.
More Information About Primary HPV Screening
Check out our primary HPV tagged materials in the Resource Library and other resources below:




